The National Agency for Food and Drug Administration Control has banned the sale of Dex Luxury bar soap due to the presence of butyphenyl methylpropional.
A statement posted on NAFDAC’s official channels on Thursday and sighted by Persecondnews, said the product, Dex Luxury Bar Soap (No. 6 Mystic Flower), manufactured in Turkey, contains BMHCA, a substance prohibited in cosmetic products due to its potential harm to the reproductive system and skin sensitization.
The post reads: “Public Alert No. 012/2024. Ban on the sale of Dex Luxury Bar Soap due to Butyphenyl Methylpropional (BMHCA) content.
“NAFDAC is notifying the public of the ban on the sale of Dex Luxury Bar Soap (No. 6 mystic flower) by the European Union (EU).
“The product does not comply with the Cosmetic Products Regulation as it is said to contain Butyphenyl Methylpropional (BMHCA), which is prohibited in cosmetic products due to its risk of harming the reproductive system, causing harm to the health of the unborn child, and may cause skin sensitization.
“As a result, a ban on the marketing of the product has been placed by some regulatory and public authorities in the EU.
“Product Name: Dex Luxury Bar Soap (No. 6 Mystic Flower), Product Brand: DEX, Country of Manufacture: Turkey, Model: 11.11.22, Barcode: 8694965531, Category: Cosmetics.
“Although this product is not on the NAFDAC database, importers, distributors, retailers, and consumers are advised to exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and use of the above-mentioned product.
“Healthcare professionals and consumers are advised to report any suspicion of adverse reactions or substandard and falsified regulated products to the nearest NAFDAC office.”
In the same vein, NAFDAC has recalled the popular cough syrup, Benylin Pediatrics, manufactured by Johnson & Johnson, following recent toxicity findings in the laboratory on the product.
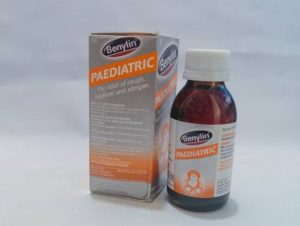
In a statement published on its website on Wednesday, the agency said laboratory analysis conducted on the product showed that it contains an unacceptable high level of diethylene glycol and was found to cause acute oral toxicity in laboratory animals.
“Benylin pediatric syrup is indicated for the relief of cough and its congestive symptoms and for the treatment of hay fever and other allergic conditions in children aged 2 to 12 years.
“Diethylene glycol is toxic to humans when consumed and can prove fatal. Toxic effects can include abdominal pain, vomiting, diarrhea, the inability to pass urine, headaches, altered mental states, and acute kidney injury, which may lead to death.
The details of the affected product showed that Benylin Pediatric was produced by Johnson & Johnson, Cape Town, South Africa.
With batch number 329304, the product was manufactured in May 2021, and it is to expire this month, April 2024.






















Leave a comment