NAFDAC Director-General, Prof. Mojisola Adeyeye, disclosed this in a statement in Abuja on Monday.
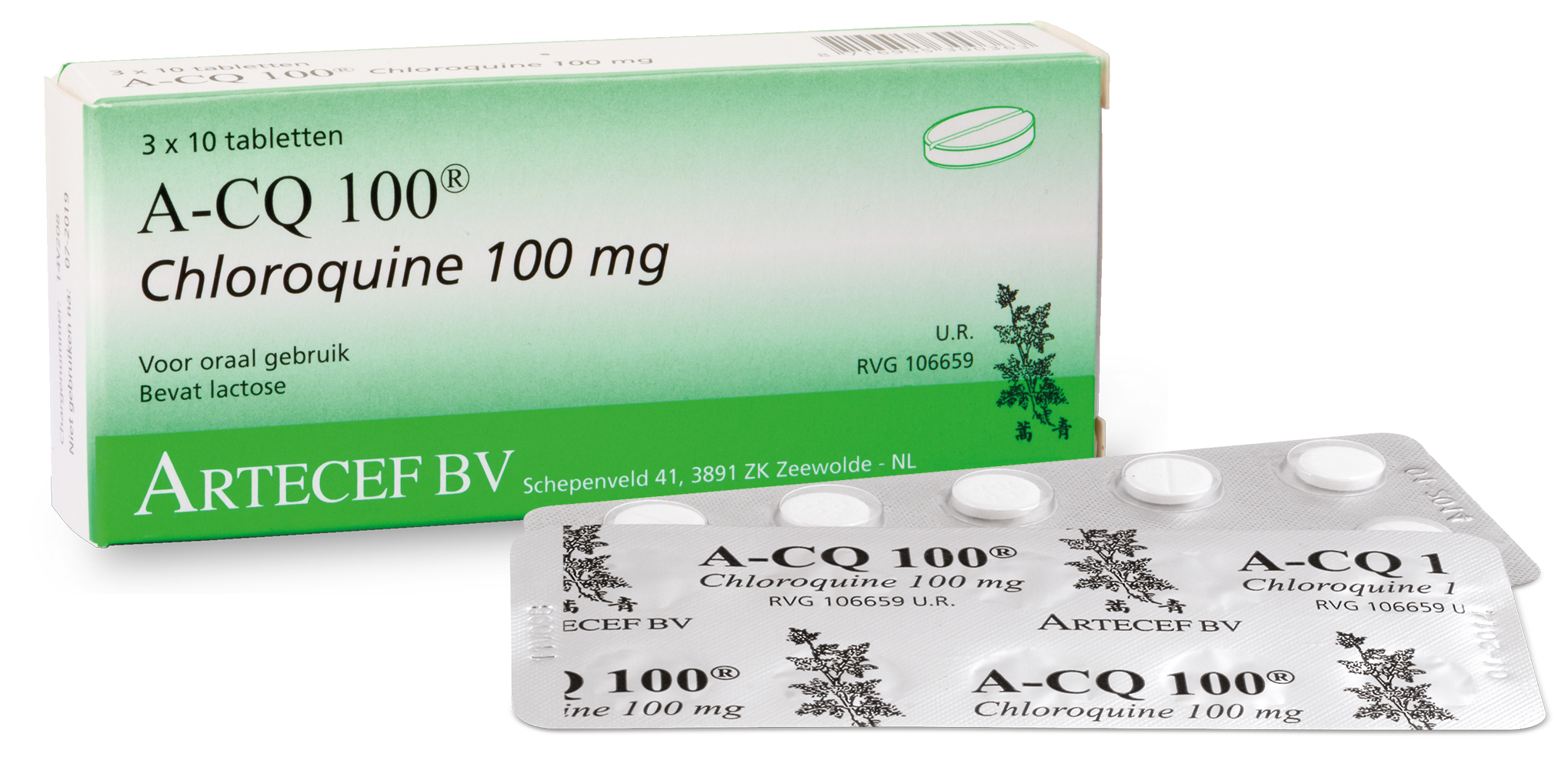
Chloroquine was reported to have demonstrated marked efficacy and acceptable safety in treating COVID-19 associated pneumonia in multi-centre clinical trials conducted in China.
The study, which involved 10 hospitals in Wuhan, Jingzhou, Guangzhou, Beijing, Shanghai, Chongqing and Ningbo, was reported to be superior to controlling pneumonia associated with COVID-19 and shortening the cause of the disease.
Nigeria discontinued the use of Chloroquine as an anti-malaria drug many years ago because of the resistance the parasite developed against the drug.
“The company had NAFDAC approval for the production of the drug as antimalarial many years ago before the discontinuation. But my fear is possible difficulty in getting the API due to the fact that the drug has been discontinued long ago.
“But a few days after, they called that they were able to get the API, and I asked them to manufacture a batch for emergency stock just in case more people become exposed and infected with the virus.
She warned Nigerians against using it without a medical doctor or clinician’s prescription for cases of clinical trial treatment of COVID-19.





















Leave a comment