U.S President Donald Trump said Thursday that he directed the Food and Drug Administration, FDA, to investigate whether an existing drug given to malaria patients (chloroquine) can also be used to treat the novel coronavirus.
There are no proven therapies for the COVID-19 virus, which has rapidly grown into a pandemic.
It is important “not to provide false hope,” FDA Commissioner Stephen Hahn said at the White House’s daily press briefing on the coronavirus. But Trump has “asked us to be aggressive” and “break through exciting, life-saving treatment, and we’re doing that at the FDA,” Hahn said.
Hahn also highlighted another experimental treatment possibility that the FDA is investing: Using plasma derived from blood taken from coronavirus patients who have recovered, and injecting that into other patients in an attempt to potentially jump start their own immune response.
“There’s a cross agency effort about something called convalescent plasma,” he said. “This is a pretty exciting area. And again, this is something that we have given assistance to other countries with as this crisis has developed, so FDA has been working for some time on this. If you’ve been exposed to coronavirus and you’re better, you don’t have the virus in your blood. We could collect the blood now this is a possible treatment. This is not a proven treatment, I just want to emphasize that, [but we would] collect the blood, concentrate that and have the ability, once it’s pathogen free, that is virus free, be able to give that to other patients and the immunoglobulins, the immune response could potentially provide a benefit to patients.”
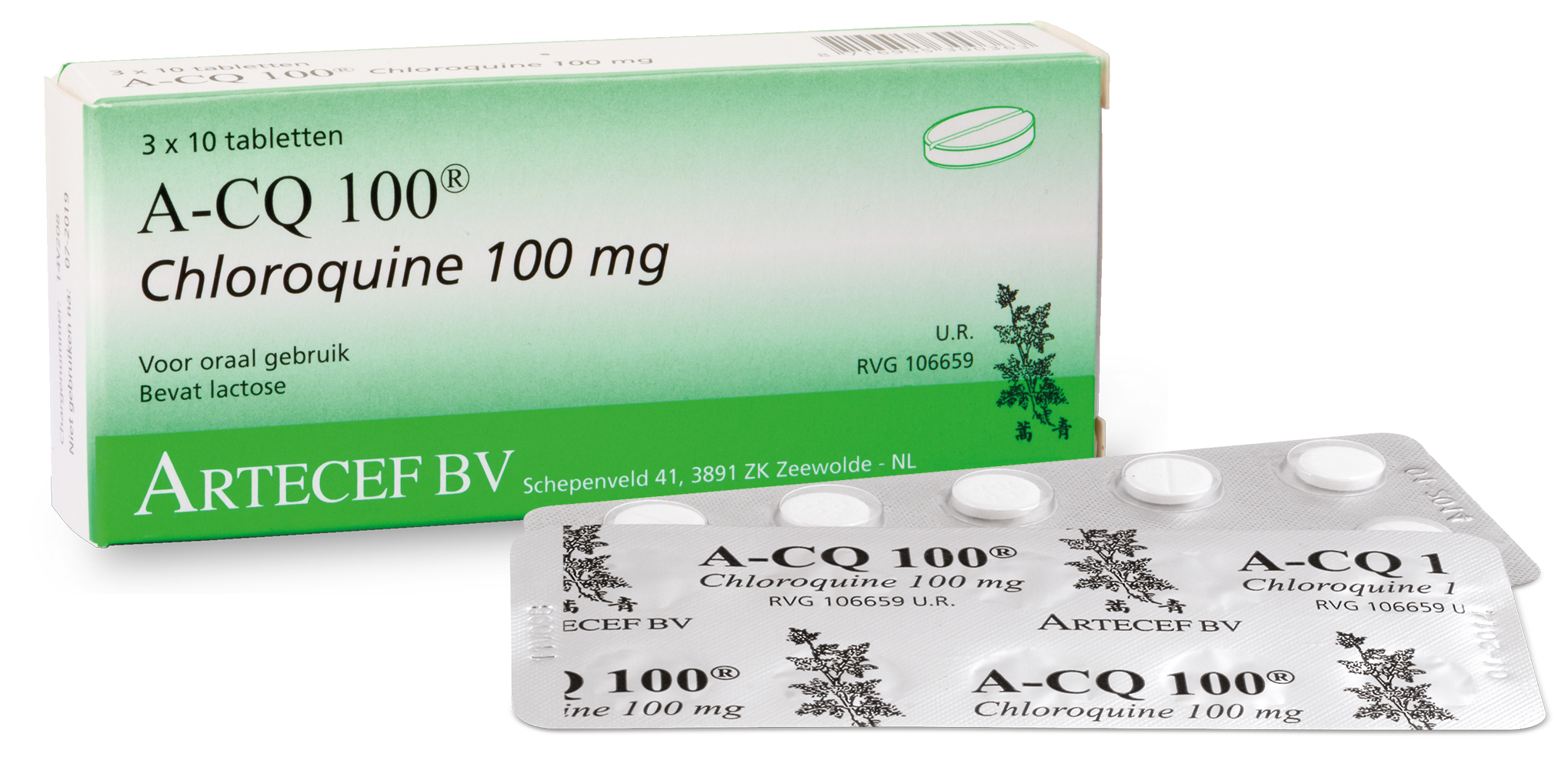
But some scientists had claimed early in the outbreak that the anti-malaria drug, Chloroquine, could be a potential treatment for the new virus.
Given chloroquine’s effectiveness in treating SARS, scientists have investigated if it will be an effective treatment against the new coronavirus responsible for COVID-19. So far, the initial trials are encouraging.
“There is evidence that chloroquine is effective when they looked at SARS in vitro with primate cells,” said Dr. Len Horovitz, a pulmonologist and internist at Lenox Hill Hospital in New York City.
“The theory of the experiment with primate cells was that chloroquine could be for preventing viral infection or as a treatment for viral infection after it had occurred. In vitro in these primate cells, there was evidence that viral particles were significantly reduced when chloroquine was used.”
“The way that it worked against SARS was by preventing of the attachment of the virus to the cells. Chloroquine interfered with the attachment to that receptor on the cell membrane surface,” Horovitz said. “So it’s disrupting a lock and key kind of mechanism of attachment.”
Researchers in China found that treating patients with COVID-19-associated pneumonia with chloroquine may shorten their hospital stay and improve the patient’s outcome.
U.S. health officials say a vaccine ready for public use could take 12 to 18 months.




































Leave a comment